Screening Area:
- Dedicated Screening Zone for volunteer intake and assessments.
- Waiting & Registration Area for volunteers.
- 2 Informed Consent Form (ICF) Rooms for privacy during consent process.
- 2 ECG Rooms for cardiovascular evaluations.
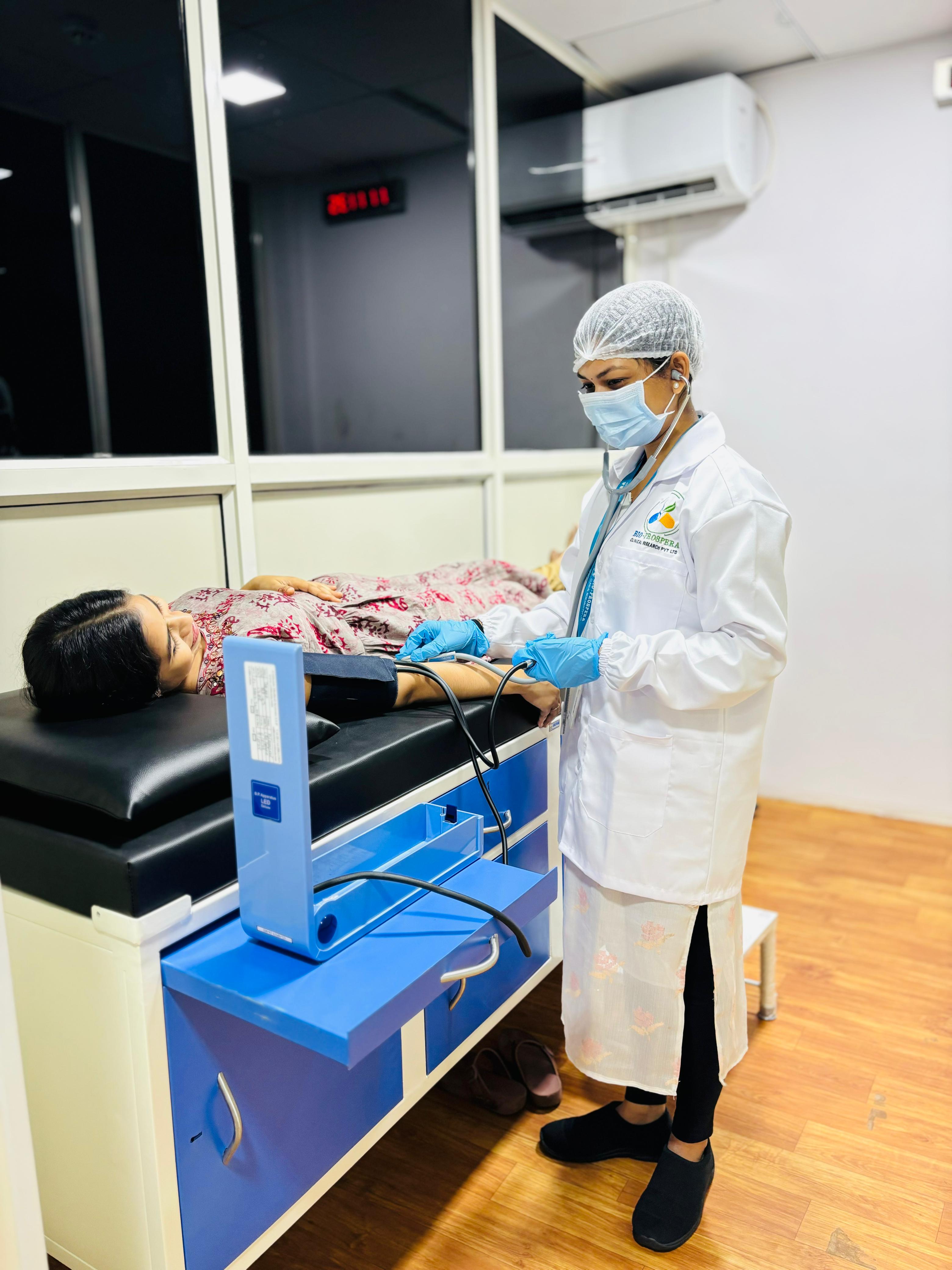
- 2 Medical Examination Rooms for initial physical assessments.
- Screening Phlebotomy Area: Equipped with yellow monochromatic light and 2 dry urinals for sample collection.
- Volunteer Information Management System (VIMS): Manages in-house volunteer database for efficient tracking.
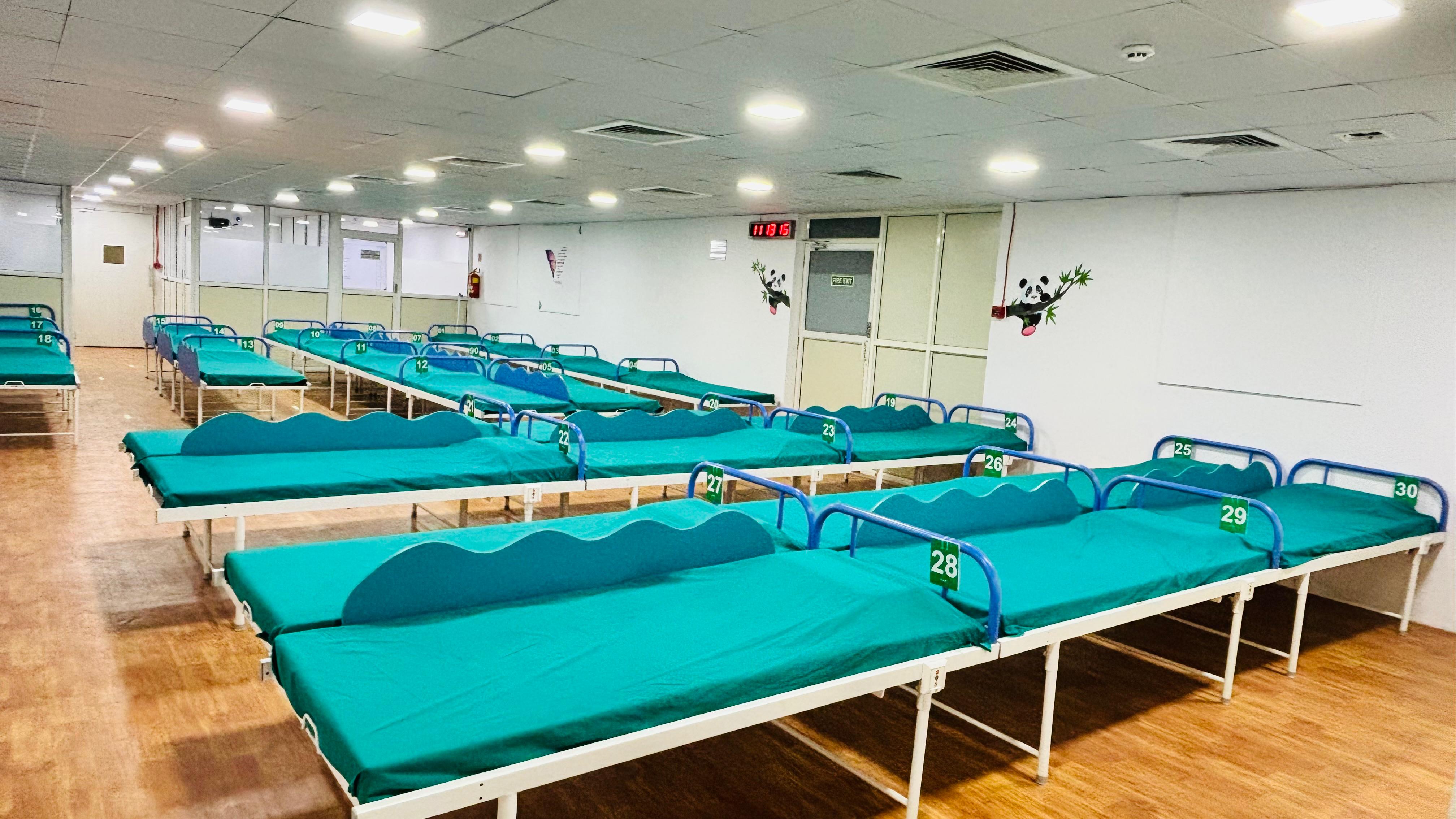
Clinical Pharmacological Unit (CPU) – 60 Beds Facility:-
- Two CPUs with 30-bed capacity each, designed for large-scale study execution.
- Dosing & Phlebotomy Area: Dedicated space for drug administration and sample collection.
- Pantry & Recreational Area for volunteer comfort.
- Light-Sensitive Molecule Handling: CPU equipped with monochromatic light for sensitive compounds.
- Sample Processing & Storage: Equipped with -20°C and -80°C secure deep freezers, and refrigerated centrifuge machines for proper storage and processing.
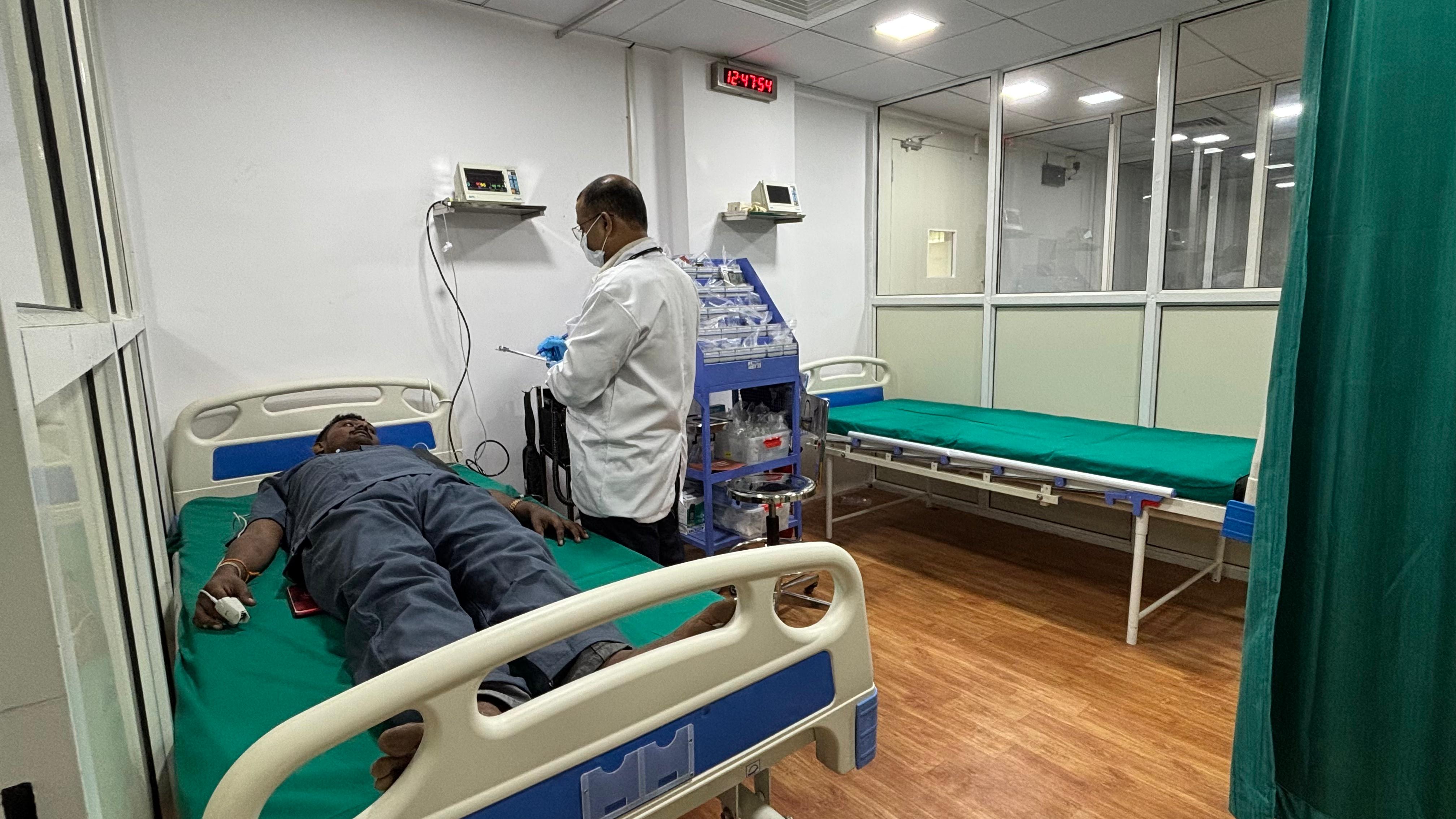
- ICU Support: Both CPUs are supported by fully functional, 2-bedded modern ICUs with emergency equipment, including patient monitors, defibrillators, and a range of oral and injectable emergency medicines.

Pharmacy:
- Centralized Drug Storage Unit: Equipped with air curtain and air shower to prevent microbial contamination.
- 21 CFR Compliant Stability Chamber: Ensures controlled temperature and humidity for the storage of investigational products.
- Refrigeration for Temperature-Sensitive Drugs: Dedicated refrigeration for storing medicines between 2°C and 8°C.
- Laminar Air Flow: Ensures aseptic dispensing procedures.
- Analytical Balance: Used for accurate weighing and suspension dispensing of investigational drugs.
Compliance & Standards:
- All procedures are carried out in strict accordance with Standard Operating Procedures (SOPs) and international regulatory guidelines to ensure the highest standards of safety and quality in BA/BE studies.
- All critical instruments, including deep freezers, stability chambers, and refrigerators, are equipped with 21 CFR Part 11-compliant dataloggers. These dataloggers continuously monitor and record temperature and humidity levels 24/7, ensuring that all conditions required for the proper storage of investigational products and samples are met at all times. This system provides real-time data and alerts, ensuring compliance with regulatory requirements and the integrity of stored materials.

Clinical SOPs:
Our clinical facility adheres to a comprehensive set of Standard Operating Procedures (SOPs) that ensure consistent, high-quality results and strict compliance with international regulatory standards, including CDSCO, US-FDA, MHRA, Health Canada, ANVISA, and other Rest of World (ROW) guidelines.
These SOPs cover every aspect of our operations, including:
- Sample Handling: Clear protocols to ensure the integrity and traceability of samples throughout the study process.
- Equipment Maintenance: Rigorous procedures for the calibration, validation, and maintenance of equipment, ensuring accurate and reliable data collection.
- Data Management: Detailed procedures for data integrity, confidentiality, and regulatory reporting, ensuring compliance with GCP (Good Clinical Practices).
In addition to maintaining the highest standards in clinical operations, our SOPs prioritize the safety and well-being of all volunteers. Every aspect of study design and execution is built to promote the comfort, care, and protection of our volunteers, ensuring a safe and ethical environment for BA/BE studies.
Experienced Clinical Team
Our clinical team is highly skilled and knowledgeable, proficient in all aspects of clinical trial execution in respect to safety and well-being of volunteers, including:
- Screening: Conducting thorough volunteer assessments to ensure eligibility for studies.
- Check-in: Ensuring smooth and accurate volunteer registration and initial evaluation.
- Dosing: Administering investigational products according to the study protocol with precise attention to detail.
- Sample Collection & Processing: Collecting, processing, and transferring samples to the bioanalytical department while maintaining the integrity and chain of custody.
Our team is not only adept at performing these critical activities with efficiency but is also highly trained in navigating regulatory audits from multiple global agencies, including CDSCO, US-FDA, MHRA, Health Canada, and ANVISA. We maintain the highest standards of clinical practice and ensure compliance with all regulatory requirements, enabling seamless audits and continuous quality assurance.
Capability of handling studies like-
- Oncology
- Female
- CNS acting
- FDC of antihypertensive
- Patch
- Injectable
- Solid/ Liquid Oral
- Inhaler